Acid Vs Base Examples In the field of chemistry they are called bases or hydroxides to substances that when dissolved in water release hydroxyl ions OH and are called acids substances that are capable of releasing protons H in aqueous solution
Acids and bases are two types of compounds that readily react with one another Acids are substances that donate protons H ions or accept electron pairs Common examples include vinegar acetic acid CH COOH citrus fruits citric acid C H O and stomach acid hydrochloric acid HCl Acids exist as solid liquid and gas based on temperature Bases usually exist in the solid state except for ammonia which exists as a gas Acids are sour in taste Bases taste bitter Acids release hydrogen ions H when dissolved in water Bases release hydroxyl ions OH when dissolved in water
Acid Vs Base Examples

Acid Vs Base Examples
https://i.ytimg.com/vi/H5fiHJDVJLI/maxresdefault.jpg
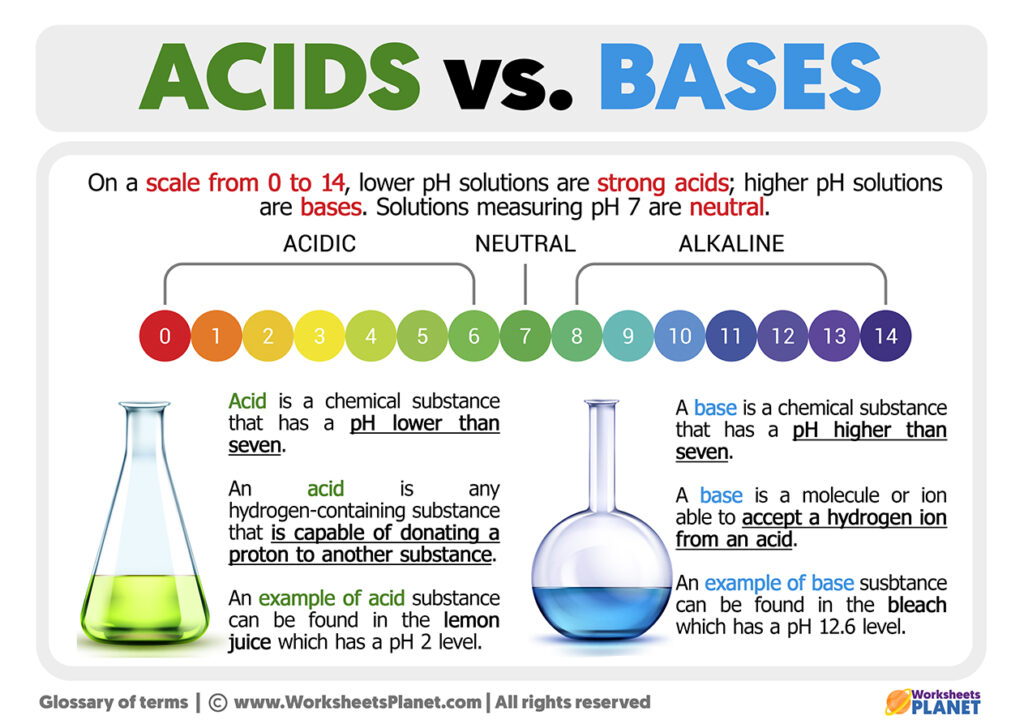
Acid And Bases Differences
https://www.worksheetsplanet.com/wp-content/uploads/2022/12/Acids-and-bases-1024x724.jpg

Lewis Vs Bronsted CHEMISTRY COMMUNITY
https://www.chemistrysteps.com/wp-content/uploads/2019/04/Lewis-acids-and-bases-vs-Arrhenius-and-Bronstead-acids-and-bases.png
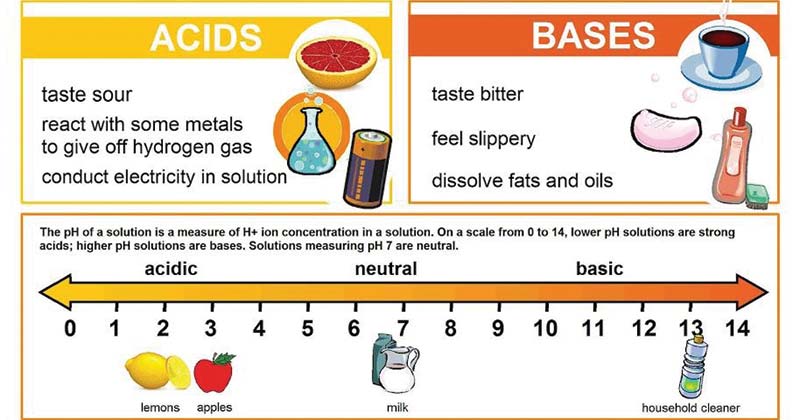
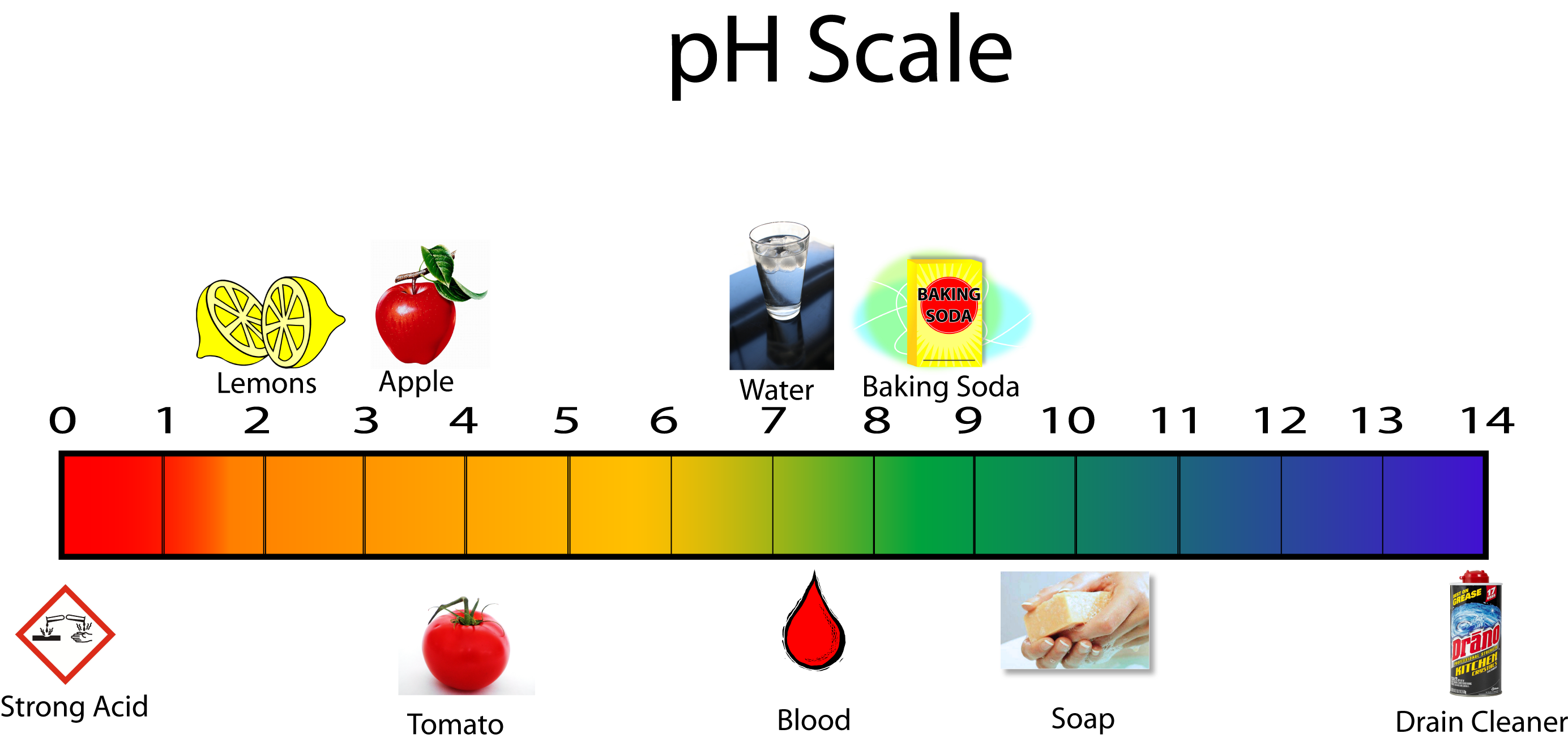
Basic alkaline substances feel soupy while acidic substances may sting The acids turn blue litmus paper to read but do not change the color of red litmus paper Bases turn red litmus paper blue but do not change the color of blue litmus paper as illustrated in Fig 6 1 2 What s the difference between Acid and Base Bases are the chemical opposite of acids Acids are defined as compounds that donate a hydrogen ion H to another compound called a base Traditionally an acid from the Latin acidus or acere meaning sour was any chemical compound that when dissolv
Acids may be defined as the compounds that donate an ion of hydrogen H to another compound usually called a base Conventionally an acid used to be known as the chemical compound that once dissolved in water produces a solution with The base is a substance that accepts an ion or a proton to form its conjugate acid and the acid is a substance that donates an ion or a proton to form its conjugate base according to the Bronsted Lowry theory
More picture related to Acid Vs Base Examples
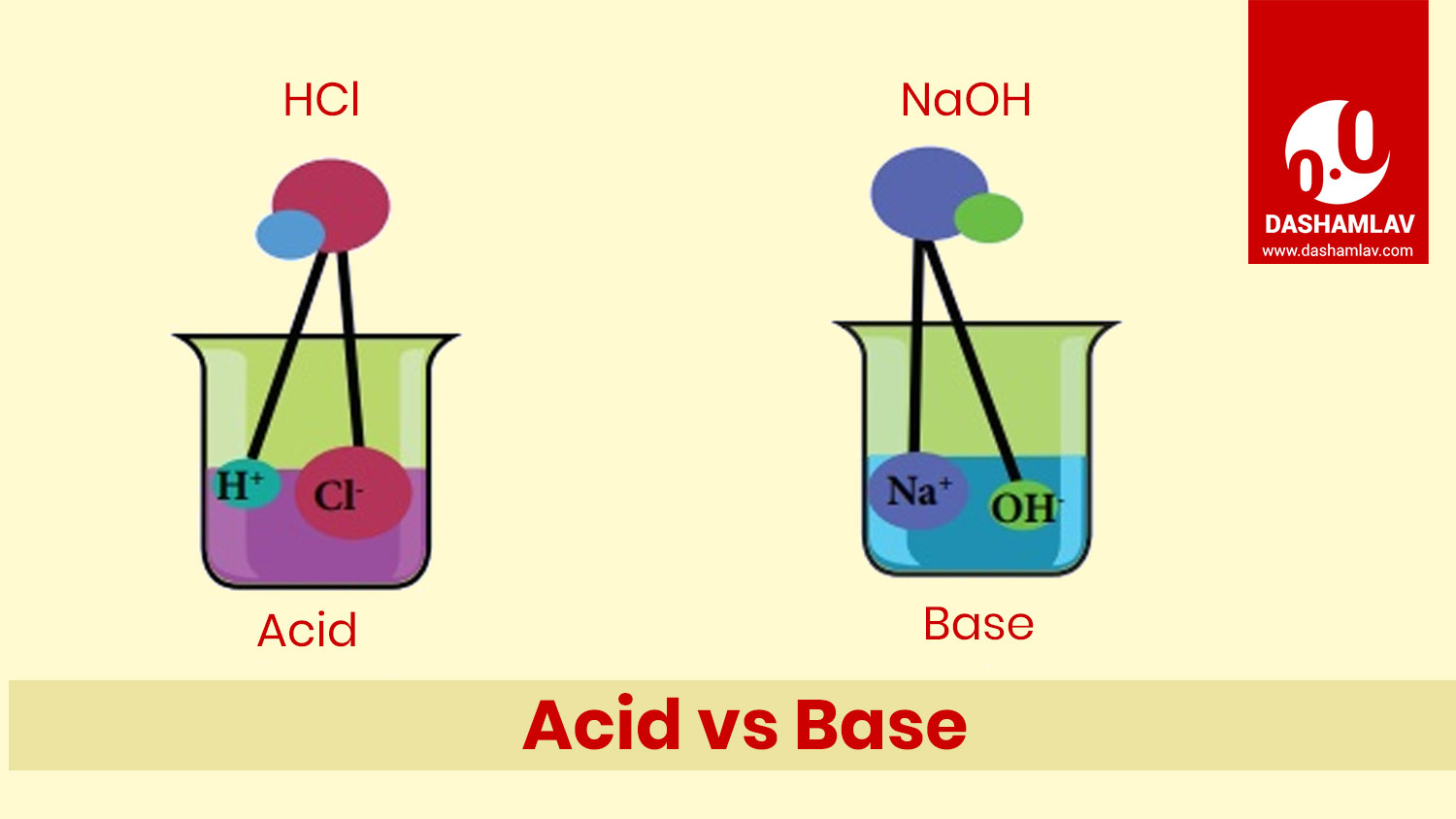
Acid Vs Base Table Of Differences Between Acid And Base
https://dashamlav.com/wp-content/uploads/2022/06/acid-vs-base-dashamlav.jpg
Acid Vs Base Definition 16 Major Differences Examples
https://scienceinfo.com/wp-content/uploads/2020/05/Differences-between-Acid-and-Base.jpg
Acids And Bases MCHS Science
http://mchsscience.weebly.com/uploads/5/6/4/7/5647812/458044735_orig.png
Acids have a pH level lower than 7 0 while bases have a pH level higher than 7 0 Discover why acids and bases have a different pH level along with other important properties by looking at each substance The term acid and base have been defined in different ways depending on the particular way of looking at the properties of acidity and basicity Arrhenius first defined acids as compounds which ionize to produce hydrogen ions and bases as compounds which ionize to produce hydroxide ions
[desc-10] [desc-11]
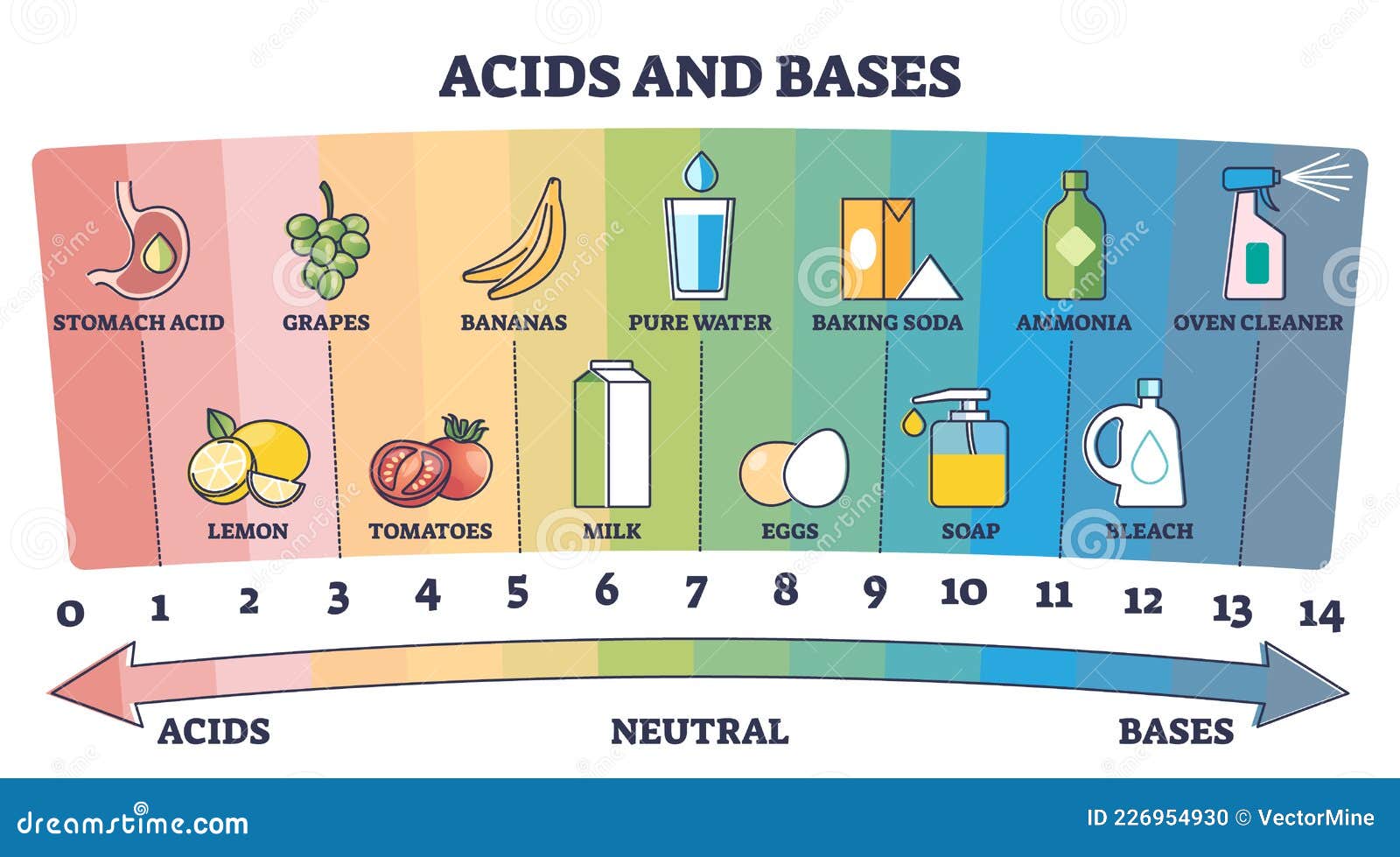
Ph Scale Acids And Alkalines Examples Stock Photo CartoonDealer
https://thumbs.dreamstime.com/z/acids-neutral-bases-substances-scale-examples-outline-diagram-acids-neutral-bases-substances-scale-examples-226954930.jpg
Acids And Bases
https://storage.googleapis.com/ltkcms.appspot.com/fs/yd/images/cover/difference-acids-bases.base?v=1594737918

https://www.exampleslab.com
In the field of chemistry they are called bases or hydroxides to substances that when dissolved in water release hydroxyl ions OH and are called acids substances that are capable of releasing protons H in aqueous solution
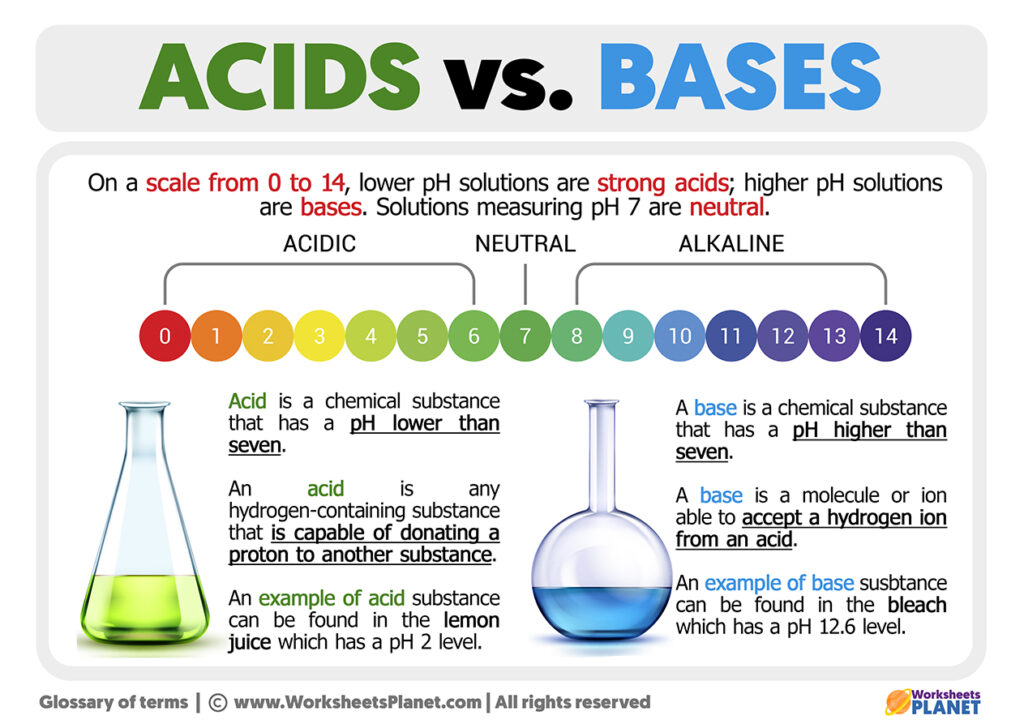
https://sciencenotes.org › acid-base-chemistry
Acids and bases are two types of compounds that readily react with one another Acids are substances that donate protons H ions or accept electron pairs Common examples include vinegar acetic acid CH COOH citrus fruits citric acid C H O and stomach acid hydrochloric acid HCl

Acids And Bases

Ph Scale Acids And Alkalines Examples Stock Photo CartoonDealer

Meghallgat s Logika Bajusz Acid Acid M g Sz p Ruha Dokk

Examples Of Bases
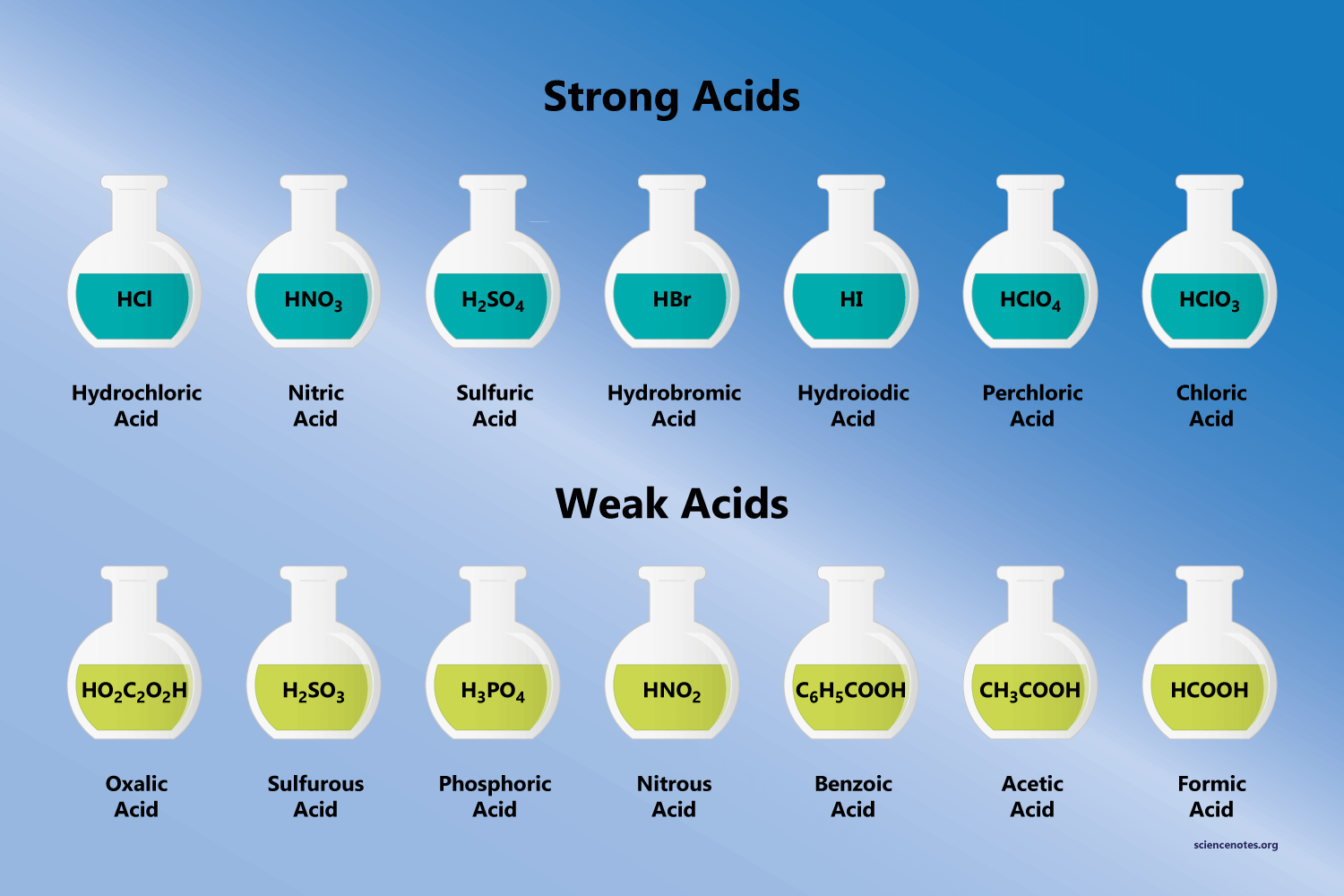
Examples Of Bases
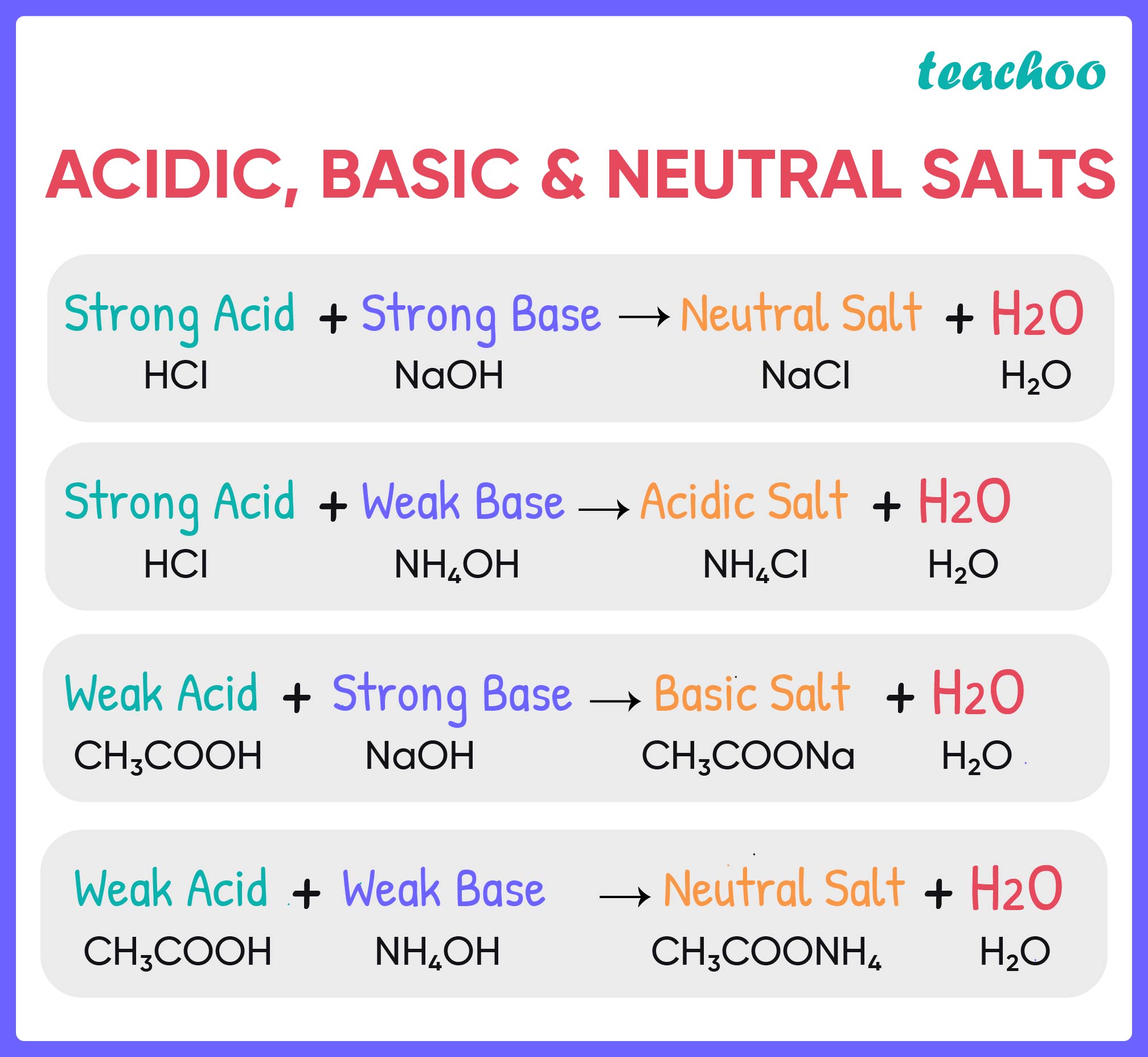
Salts And It s Properties with Examples Acids Bases And Salt

Salts And It s Properties with Examples Acids Bases And Salt

Acids And Bases List

Strong And Weak Acids And Bases Definition Examples Expii
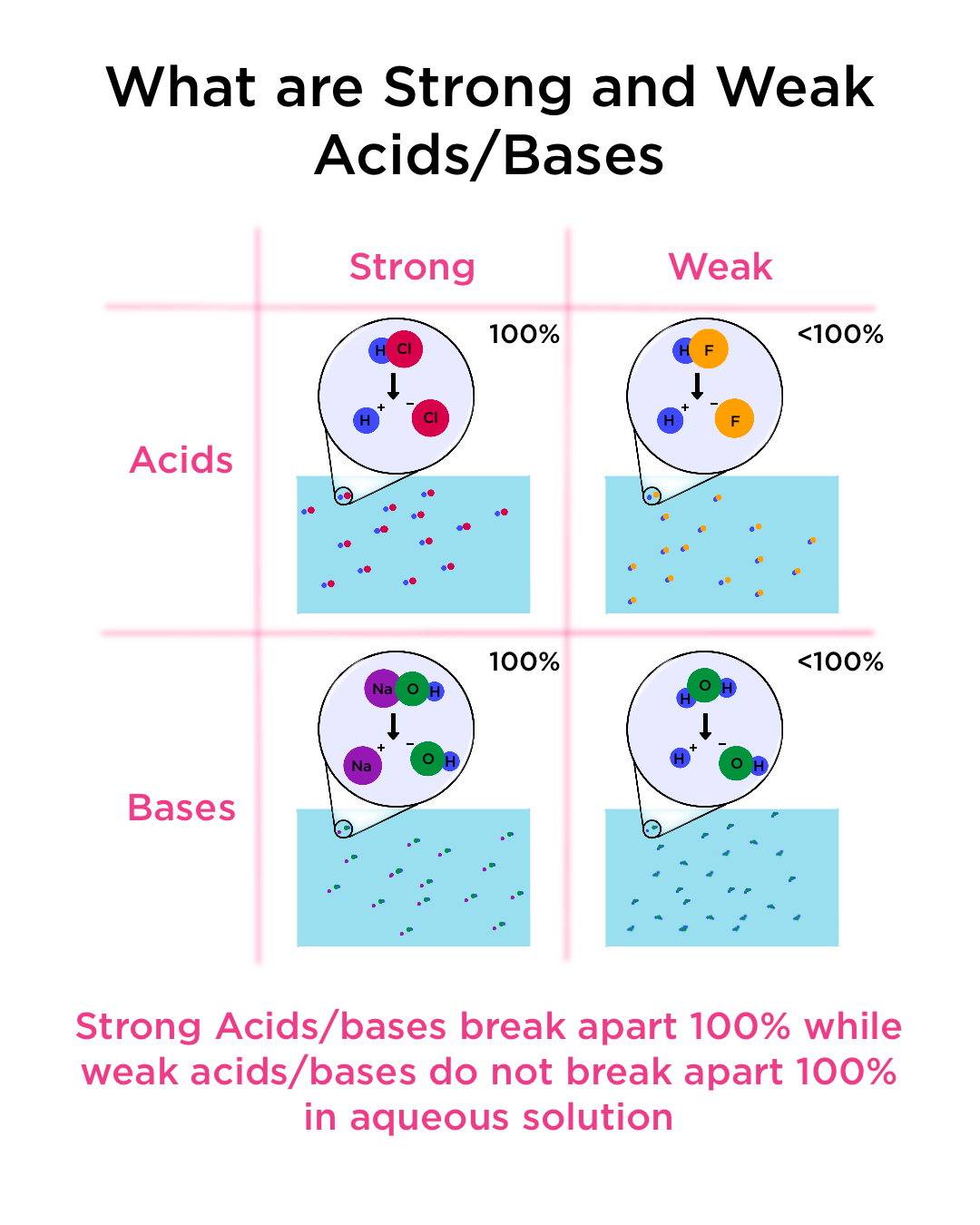
Strong And Weak Acids And Bases Definition Examples Expii
Acid Vs Base Examples - [desc-13]
